Proton-pump inhibitors (PPIs) like omeprazole are routinely prescribed for conditions like heartburn and inflammation or ulceration of the stomach or intestines.
But their long-term use has been associated with adverse issues, including gastrointestinal infections, microscopic stomach and intestinal inflammation, malabsorption of vitamins and minerals, bone fractures, kidney disease, and a potential increase in gastric and other cancers. These drugs are also associated with alterations in the gut microbiome.
Studies show that many people currently taking PPIs do not have an indication for remaining on these meds.
New guidelines from the American Gastroenterological Association recommend that doctors should consider de-prescribing PPIs for any patient without a clear indication for taking these meds. When indicated, patients should be treated with the lowest PPI dose required.
(Targownik et al 2022)
If you’re on a PPI, ask your doctor to:
–> document the indication for a PPI prescription
–> figure out if you’re a candidate for de-prescribing
–>advise you on how de-prescribing should happen, in order to minimize withdrawal symptoms
As always, ensure you’re optimizing dietary and lifestyle factors for heartburn or other GI conditions.
More detailed recommendations from the paper:
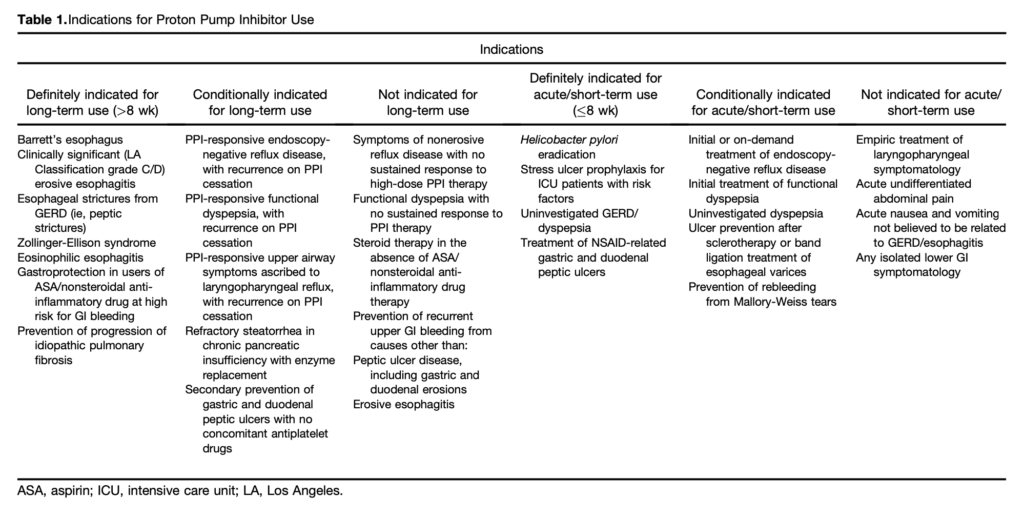
- All patients taking a PPI should have a regular review of the ongoing indications for use and documentation of that indication. This review should be the responsibility of the patient’s primary care provider. [see table 1]
- All patients without a definitive indication for chronic PPI should be considered for trial of de-prescribing.
- Most patients with an indication for chronic PPI use who take twice-daily dosing should be considered for step down to once-daily PPI.
- Patients with complicated gastroesophageal reflux disease, such as those with a history of severe erosive esophagitis, esophageal ulcer, or peptic stricture, should generally not be considered for PPI discontinuation.
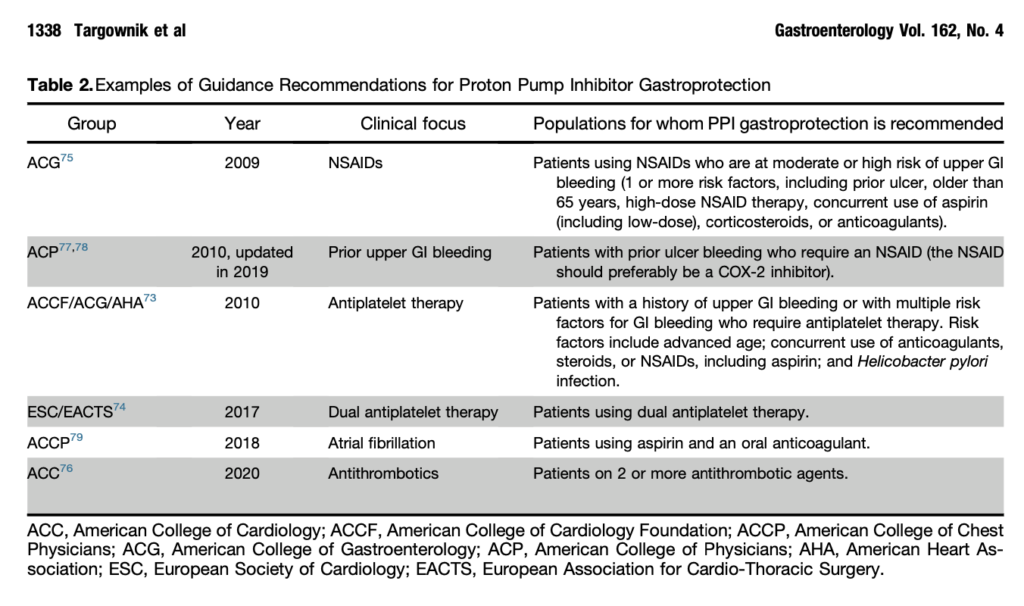
- Patients with known Barrett’s esophagus, eosinophilic esophagitis, or idiopathic pulmonary fibrosis should generally not be considered for a trial of de-prescribing. [see table 2]
- PPI users should be assessed for upper gastrointestinal bleeding risk using an evidence-based strategy before de-prescribing.
- Patients at high risk for upper gastrointestinal bleeding should not be considered for PPI de-prescribing.
- Patients who discontinue long-term PPI therapy should be advised that they may develop transient upper gastrointestinal symptoms due to rebound acid hypersecretion.
- When de-prescribing PPIs, either dose tapering or abrupt discontinuation can be considered. [For example, slowly taper off the PPI over 2-4 weeks, and let patients know they will likely have reflux symptoms for a couple of weeks after they stop the medication. Continue to implement lifestyle interventions during this time.]
- The decision to discontinue PPIs should be based solely on the lack of an indication for PPI use, and not because of concern for PPI-associated adverse events (PAAEs). The presence of a PAAE or a history of a PAAE in a current PPI user is not an independent indication for PPI withdrawal. Similarly, the presence of underlying risk factors for the development of an adverse event associated with PPI use should also not be an independent indication for PPI withdrawal.